Detection of bacterial biofilms using covalent lectin binders
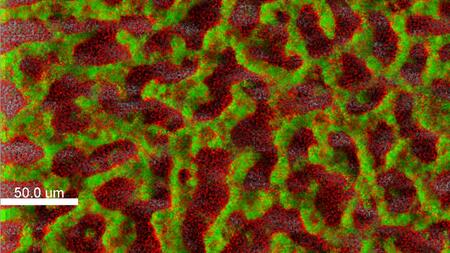
Biofilm of a clinical isolate of Pseudomonas aeruginosa stained with fluorescent dyes (green: living cells; red: dead cells)
DZIF researchers from the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) produced a molecule that provides a way to visualise Pseudomonas infection. The scientists published their results in the international edition of Angewandte Chemie.
Pseudomonas aeruginosa is a major pathogen that can cause severe infections in various organs of the human body. The infection becomes particularly harmful when the bacteria agglomerate into biofilms: They do this not only to evade attacks of the immune system, but also to protect themselves from the effects of antibiotics. This results in chronic infections that are almost impossible to treat. Scientists from the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), a joint branch of the Helmholtz Centre for Infection Research (HZI) and Saarland University, developed a molecule, which binds specifically and irreversibly to a key bacterial protein in biofilms. By binding, this molecule blocks an important component of the biofilm and can also visualise the biofilm, when it is labelled with an appropriate dye.
Bacteria of the Pseudomonas aeruginosa species are hospital germs and are resistant to many common antibiotics. They can infect all organs and implants and form so-called biofilms, in which they are protected and survive for long periods of time. This results in a chronic infection that is very difficult to treat, since both the immune system and antibiotics cannot harm the bacteria. To produce a biofilm, Pseudomonas bacteria release certain proteins called lectins. These proteins bind to sugar molecules outside the bacterial cells and cross-link them to form a matrix, in which the pathogens agglomerate. If it was possible to block these lectins with an agent, existing biofilms could be dissolved or the production of biofilms could be prevented altogether.
Since, for instance, the lectin called "LecA" binds sugar molecules only relatively loosely, the HIPS researchers directed by Dr Alexander Titz imparted on a search for a molecule that permanently binds to LecA. This requires a so-called covalent bond or atomic bond. "Initially, we used X-ray structural analysis to analyse the three-dimensional structure of LecA and then we rationally designed a small molecule, whose properties should allow it to form a covalent bond to the sugar binding site of LecA," says Alexander Titz. After designing this molecule, the researchers then produced it by synthesis in the laboratory and exposed it to LecA. Using a variety of biophysical methods, their collaboration partners at the HIPS and the Centre national de la recherche scientifique in Grenoble were able to prove the exact position of the binding site on the lectin. Using mass spectrometry, they were able to unequivocally demonstrate covalent binding.
"This is the first time a specific molecule was produced that binds covalently to a lectin," says Titz, who is the head of a junior research group of the HZI and the German Center for Infection Research. One possible approach to an application is to develop the molecule into an agent that suppresses biofilm production in Pseudomonas aeruginosa. The bacteria would then grow normally, but would no longer produce a biofilm and would be much less hazardous – since they lose their virulence. They would then be susceptible to treatment with antibiotics, if they are not eliminated earlier by the immune system. However, the new molecule still needs to be optimised more for this approach as a pathogenicity blocker.
"We already demonstrated another application, though, namely in imaging," says Alexander Titz. "We coupled the lectin-binding molecule to a fluorescent dye and were able to visualise existing biofilms." To date, a diagnosis of a Pseudomonas aeruginosa infection can be made, but the exact site of the infection in the body is often difficult to determine. As a result the therapy cannot be directed specifically at the affected organ or tissue. "We still need to develop the molecule further for diagnostic application in humans. For example, instead of the fluorescence dye, we need to use a probe that facilitates detection by magnetic resonance or positron emission tomography," says Titz. In addition, the long-term survival of the molecule in the body needs to be assured. These optimisation processes are ongoing in the scope of funding from the European Research Council (ERC).
Helmholtz Centre for Infection Research:
Scientists at the Helmholtz Centre for Infection Research (HZI) in Braunschweig, Germany, are engaged in the study of different mechanisms of infection and of the body’s response to infection. Helping to improve the scientific community’s understanding of a given bacterium’s or virus’ pathogenicity is key to developing effective new treatments and vaccines. www.helmholtz-hzi.de/en
German Center for Infection Research:
At the German Center for Infection Research (DZIF), about 500 scientists from 35 institutions nationwide jointly develop new approaches for the prevention, diagnosis and treatment of infectious diseases. Their aim is to translate research results into clinical practice rapidly and effectively. With this, the DZIF paves the way for developing new vaccines, diagnostics and drugs in the fight against infections. Further information at: www.dzif.de