First clinical trial of DZIF vaccine against COVID-19 gets go-ahead
The Paul-Ehrlich-Institut has approved the clinical phase I trial of the vaccine at the German Center for Infection Research.
The DZIF vector vaccine against Corona enters clinical trials again.
The Paul-Ehrlich-Institut, the Federal Institute for Vaccines and Biomedicines, today approved the clinical trial of the MVA-SARS-2-S vaccine against COVID-19. The vector vaccine was developed by scientists at the German Center for Infection Research and IDT Biologika GmbH. In the first clinical phase, it is being examined for safety, tolerability and its specific immune response against the pathogen. The clinical trial is due to start with the recruitment of the first of a total of 30 trial participants in October at the medical contract institute, CTC North, University Medical Centre Hamburg-Eppendorf.
“I congratulate the entire DZIF vaccines team on the rapid development of a promising candidate for a vaccine against SARS-CoV-2. This was only possible through close cooperation between university institutes and clinics at several DZIF locations, together with our industry partner. We are pleased that the first clinical trial has been approved and will begin very soon, and we are naturally hoping for good results,” said Prof. Dr. Hans-Georg Kräusslich, Chairman of the Board of the German Center for Infection Research.
It is a vector vaccine against the SARS-CoV-2 virus developed at the Ludwig Maximilian University of Munich (LMU), in which the genetic information for a surface protein of the SARS-CoV-2 virus is incorporated in a modified and thus harmless smallpox virus (MVA). This viral vector cannot replicate, but the introduced DNA sequence – the coronavirus component – can simulate an infection and trigger the production of COVID-19 antibodies and immune cells.
“We are pleased about the Paul-Ehrlich-Institut’s approval. In the last few months, we developed the vaccine together with our DZIF cooperation partners, Prof. Dr. Gerd Sutter from LMU and Prof. Dr. Stephan Becker from the Philipps University of Marburg. We are now reviewing its efficacy and safety,” said Prof. Dr. Marylyn Addo, Head of Infectious Diseases at the University Medical Centre Hamburg-Eppendorf (UKE) and lead investigator in the clinical trial.
The MVA vaccine virus was generated at LMU as a safe vaccine against smallpox over 30 years ago, and has already been used successfully in the development of a vaccine against the MERS coronavirus. IDT Biologika GmbH, a company that produces biotechnological vaccines and pharmaceuticals, has now developed a process for the large-scale production of high-purity MVA vector vaccines in a cell line and has already completed the production and filling of the vaccine doses for the first human clinical trial. Preclinical models at the Universities of Marburg and Munich have already shown that the MVA vector vaccine elicits the desired immune responses and a protective effect against SARS-CoV-2.
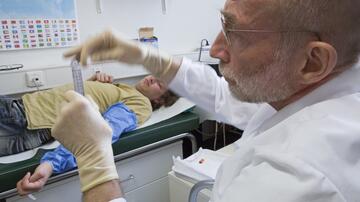
The trial will start with preliminary examinations and the selection of healthy volunteer test subjects in October. The first test subject will initially receive a low dose of the vaccine. For safety reasons, only one test subject will be vaccinated initially, and the next two not until 24 hours later. In total, the 30 test subjects aged between 18 and 55 will be vaccinated in eight groups and two increasing doses.
The study participants will receive two vaccinations every four weeks and will be medically monitored for a few hours after each vaccination at CTC North, the institute specialising in the early phases of clinical trials. On the days following the vaccinations and in the course of the following six months, the test subjects will have to attend regular outpatient follow-up examinations in order to assess vaccine tolerance, possible side effects and the immune response using blood tests and surveys. The scientists in Professor Addo's working group and at the DZIF partners in Marburg will simultaneously measure the formation of antibodies and T cells in the body and compare them with the immune responses of recovered COVID-19 patients.
“If the results of the phase I trial show a good safety profile and good vaccine-induced immune responses, a larger phase II clinical trial is planned for the end of the year. We will include additional groups of test subjects in this trial phase, including older people,” Professor Addo explains. In addition to the UKE, the university clinics in Tübingen, Marburg and Munich as well as other partner institutions will be involved in these phase II trials.
DZIF press office contact
Karola Neubert and Janna Schmidt
presse@dzif.de
UKE press office contact
Anja Brandt
anja.brandt@uke.de