How the coronavirus damages blood vessels in the brain
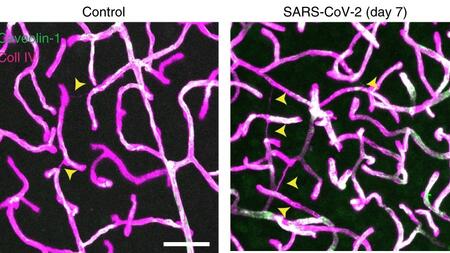
The brain accumulates dead blood vessels after SARS-CoV-2 infection (yellow arrowheads, results from mice).
SARS-CoV-2 affects not only lungs and airways, but influences many organs of the human body. How it damages small blood vessels in the brain was deciphered by a consortium of the German Centers for Lung Research (DZL), Infection Research (DZIF) and Cardiovascular Research (DZHK). They recently published their results in Nature Neuroscience.
SARS-CoV-2 coronavirus enters the body via the respiratory tract and lungs and airways are the obvious center of the disease symptoms. However, it was shown early on that many other organs and the blood vessels are also affected. For example, neurological symptoms can occur in both the acute and late phases of the disease. These include frequent anosmia or epileptic seizures, strokes, loss of consciousness, and confusion. How coronavirus triggers these cognitive and psychiatric symptoms is largely unclear. Results are conflicting, if the coronavirus directly attacks brain cells. What is known, however, is that it attacks blood vessels – including those in the brain. Against this background, an international consortium led by the Lübeck pharmacologist Prof. Markus Schwaninger (DZHK) asked what mechanism underlies the brain damage. To this end, the group focused on the small blood vessels and the endothelial cells that line them.
How the coronavirus damages the brain
The study results show that the coronavirus can indeed enter the cell via the ACE2 receptor produced by some endothelial cells and trigger a characteristic pathology that can be seen under the microscope. In endothelial cells, the viral enzyme Mpro destroys the endogenous protein NEMO, triggering a cell death program. A key finding of the study is that endothelial cells and blood-brain barrier are destroyed in this way. The scientists thus discovered for the first time a mechanism by which SARS-CoV-2 directly damages microvessels in the brain. The viral enzyme Mpro could be genetically engineered for the experiments by Prof. Rolf Hilgenfeld and his team at the University of Lübeck. Hilgenfeld already determined its three-dimensional structure in March 2020 and is developing antiviral drugs at DZIF that can block the enzyme.
Possible therapeutic pathways elucidated
Interestingly, the researchers found a way to block the cell death mechanism by investigating another protein involved in this process, RIPK1. If RIPK1 is blocked in animal experiments, the endothelial cells don’t die. The entire cell death program is thus deactivated – regardless of whether NEMO was cleaved by the viral enzyme Mpro or not. RIPK1-blocking compounds are already in the early stages of clinical testing. “The results of our study suggest that such drugs could alleviate neurological long covid symptoms,” says Markus Schwaninger.
Successful cooperation of several German Centers for Health Research (DZG)
For the study, researchers from the German Centers for Lung Research (DZL), Infection Research (DZIF) and Cardiovascular Research (DZHK) collaborated in an interdisciplinary manner. They used different animal models as well as samples from Covid-19 patients. An impressive number of different methodologies were used.