Joint position statement on the monkeypox outbreak in Germany
Joint position statement on the monkeypox outbreak in Germany by the medical scientific societies DAIG, DGI, DGPI and GfV, by the professional association dagnä and the DZIF in coordination with STIKO
Rapidly growing numbers of monkeypox virus infections have been recorded on at least four continents. Therefore, rapid and consistent action is needed to limit the outbreak. Initially, the best way to approach this issue is through target group-specific education and information (awareness programme), isolation of infection cases, quarantine for close contacts and suspected cases as well as risk minimisation in interpersonal contacts. The key is to quickly evaluate whether and how vaccination can help to limit the outbreak. Approved therapeutics should be made available for potentially severe cases.
Background
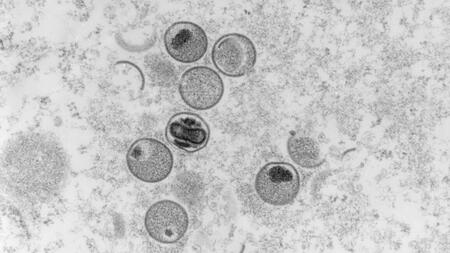
Since the diagnosis of the first case of monkeypox in London, other cases have been discovered in rapid succession on four continents. The first case in Germany was diagnosed on 19 May 2022. By 24 May 2022, about 250 confirmed global cases had already been reported to the World Health Organisation (WHO). So far, the infections have mainly appeared in younger men who reported having sex with men, but there have also been intrafamilial transmissions. Consistent with the known history of the poxvirus, which originates from West Africa, the clinical course of the disease has been relatively mild so far. The transmission route is assumed to be direct skin or mucosal contact or droplet infection. The current incidence of infection is dynamic with increasing numbers of cases. Due to the long incubation period of 1–3 weeks, assessing the extent of the outbreak as well as tracing contact chains is challenging.
Problem statement
Decisive, rapid and concerted action is needed to effectively contain the global outbreak, break the chains of infection and prevent transmission into the animal kingdom outside known endemic areas.
Proposed measures
- Outbreak management through non-pharmacological interventions
Due to the currently observed direct human-to-human transmission, target group-specific and lifestyle-accepting education and information (‘awareness’ among potentially affected persons and medical staff) is crucial. In particular, persons who have not been vaccinated against smallpox and do not have a vaccination certificate or vaccination scars should avoid contact with changing sexual partners as well as sharing beds and clothing.
Individuals with confirmed infection should remain in effective isolation for a period of 21 days. Inpatient hospitalisation for isolation purposes only is not required; inpatient admissions should primarily be for medical reasons in case of severe clinical progression or impending complications.
Contact persons with a relevant risk of infection and suspected cases should be quarantined during the incubation period or until the infection has been ruled out with certainty.
Medical staff should wear suitable protective clothing (mask, gloves, gown) when caring for the aforementioned groups of people.
- Vaccinations
A non-replicative smallpox vaccine (MVA-BN) is licensed in the EU to protect against smallpox infection (variola major, orthopoxvirus) in adults. Based on experimental animal data, this vaccine is also approved for the prevention of monkeypox in the USA and Canada. Clinical efficacy data in humans could not be collected to a sufficient extent due to the fact that cases of infection have only been sporadic so far. A corresponding study has been underway for some time. Tolerability and safety data as well as dosage recommendations in risk groups are available.
In non-pox vaccinated birth cohorts (such as birth cohorts from the early 1970s onwards), vaccination could significantly contribute to increasing protection against infection and disease. Especially in the vicinity of known infection clusters, vaccination could prevent infections or mitigate disease progression and considerably limit outbreaks.
This option should be examined promptly by the European Medicines Agency EMA (EU approval) and the STIKO (vaccination recommendation). In parallel, the availability should be checked and the procurement of vaccines in sufficient quantities as well as the organisation or feasibility of vaccination recommendations should be prepared. Established infectious disease treatment centres and the public health service should be scheduled for possible implementation.
- Examination of treatment options
Although cases to date are characterised by a known mild course of West African monkeypox virus infection, treatment options should be available for vulnerable patient populations (e.g. relevant immunodeficiency). Tecovirimat, an antiviral drug, is currently approved in the EU for the treatment of monkeypox infection; an alternative is the unapproved antiviral drug Brincidofovir. Availability of both drugs must be ensured. Treatment should also be provided through established infectious disease treatment centres.
More detailed information:
Case definition: https://www.ecdc.europa.eu/en/publications-data/risk-assessment-monkeypox-multi-country-outbreak
Clinical description: https://www.cdc.gov/poxvirus/monkeypox/index.html
Diagnosis (in German): https://www.rki.de/DE/Content/Infekt/NRZ/Konsiliar/konsiliar_pockenviren.html;jsessionid=08A34C9ADF921F35DC505BDB800049AE.internet061?nn=2386228
FAQ (in German): https://www.rki.de/SharedDocs/FAQ/Affenpocken/affenpocken_gesamt.html
https://eacademy.escmid.org/escmid/2022/monkeypox-outbreak/363235
https://www.aidshilfe.de/Monkeypox
Participating medical societies and the representatives
German AIDS Society (DAIG): Priv.-Doz. Dr. med. Christoph Boesecke, Priv.-Doz. Dr. med. Stefan Esser
German Society of Infectious Diseases (DGI): Priv.-Doz. Dr. med. Christoph D. Spinner, Prof. Dr. med. Bernd Salzberger, Prof. Dr. med. Leif-Erik Sander
German Society for Pediatric Infectious Diseases (DGPI): Prof. Dr. med. Tobias Tenenbaum
German Center for Infection Research (DZIF): Prof. Dr. med. Marylyn M. Addo, Prof. Dr. med. vet. Gerd Sutter
Society for Virology (GfV): Prof. Dr. med. Ulrike Protzer, Prof. Dr. rer. nat. Dr. med. h.c. Ralf Bartenschlager
German Study Group of Physicians in Private Practice Treating HIV-Infected Patients (dagnä): Dr. med. Axel Baumgarten, Priv.-Doz. Dr. med. Markus Bickel
Standing Committee on Vaccination (STIKO): Prof. Dr. med. Thomas Mertens