Parkinson's drug as basis for new anti-infectives
Researchers discover promising starting point for the development of new active substances against the critical pathogen Pseudomonas aeruginosa
When the bacterial pathogen Pseudomonas aeruginosa infects the body, it uses the sugar-binding protein LecA to attach itself to human cells, invade them, and form so-called biofilms. LecA thus plays a central role in the development and progression of infections with this pathogen, which is classified as particularly critical by the WHO. A research team at the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), led by Alexander Titz, a scientist at the German Center for Infection Research (DZIF), discovered that the approved Parkinson's drug tolcapone can specifically inhibit LecA activity. This discovery allows the researchers to develop new strategies for combating Pseudomonas infections. The team published their findings in the journal Angewandte Chemie International Edition.
Pseudomonas aeruginosa—one of the most common pathogens causing healthcare-associated infections—has sophisticated mechanisms to efficiently infect the human body and evade the immune system or antibiotics. With the help of the LecA protein, it can attach itself to complex sugar structures on human cells and then infect them. At the same time, the sugar-binding lectin plays a central role in the formation of gel-like biofilms, in which the pathogen is difficult to access for immune cells and antibiotics and is therefore resistant. If the activity of LecA can be restricted, for example by a pharmaceutical agent, the pathogen loses its disease-causing properties and can be more easily killed by existing antibiotics. Such agents are also known as virulence blockers and offer the advantage of exerting less selection pressure on the pathogen, which delays and potentially even prevents resistance development. A team led by HIPS group leader Alexander Titz has now identified a new starting point for the development of such virulence blockers in the already approved Parkinson's drug tolcapone. HIPS is a site of the Helmholtz Centre for Infection Research (HZI) in collaboration with Saarland University.
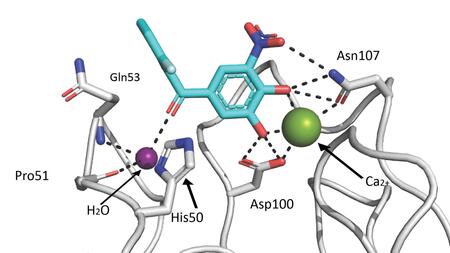
The starting point for the current study was the observation that certain catechol compounds can bind weakly to LecA. Tolcapone, a COMT inhibitor that has been approved for the treatment of Parkinson's disease for many years, was particularly effective at blocking the activity of LecA.
"Catechols are known to deliver a supposedly positive result in numerous tests. However, this is often only due to non-specific interactions that cannot be used for the development of active substances," says Titz, head of the "Chemical Biology of Carbohydrates" group and holder of the German Center for Infection Research (DZIF)-funded professorship for Organic and Pharmaceutical Chemistry at Saarland University. "However, in extensive testing, we found that tolcapone interacts with LecA via a clearly defined binding mode. That was the starting point for us to take a closer look."
By analyzing the crystal structure of LecA together with tolcapone, the team, in collaboration with the French research center CERMAV (Centre de Recherches sur les Macromolécules Végétales, Grenoble, France), was able to show that the Parkinson's drug binds to the same site on the protein as natural sugar structures. Building on these findings, the team tested over 3,200 substances from a drug library belonging to the pharmaceutical company Roche for their ability to bind to LecA. In doing so, the researchers identified several derivatives that inhibit LecA significantly more strongly than tolcapone. Some of these are even as effective as classic sugar ligands, which have been the gold standard in LecA research to date. In future studies, the scientists want to optimize the most promising candidates for use as virulence blockers against infections with P. aeruginosa. Since the molecules identified in the screening have not yet been considered for such an application, the researchers see great potential for further improvements.
Source: Press release of the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS)