SARS-CoV-2: A computer model to visualise possible attack points of viruses and virus mutants
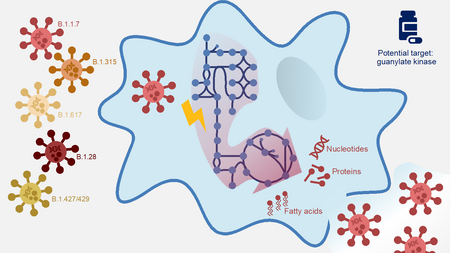
The Coronavirus attacks human cells and forces the host cells to form new virus components like nucleotides, proteins and fatty acids. A computer model identified the human guanylat kinase as a possible target for different virus mutants.
There is still an urgent need for drugs against the new coronavirus. This urgency increases further with the emergence of new virus mutations that could push vaccines to their limits. For more than a year, the group around Tübingen’s DZIF scientist Andreas Dräger has worked on a computer model that identifies weaknesses of the virus and, thus, potential attack points. Previously, the researchers have already identified a human enzyme as such a promising target. Their latest study confirms this result. Furthermore, the bioinformaticians show new attack points and demonstrate that they also apply to the new mutants.
“Even at the start of the year, we had already seen in our model that one human enzyme – guanylate kinase 1 – is essential for the replication of the virus,” Dr Andreas Dräger explains. If this kinase is switched off, the virus can no longer proliferate. “However, this process does not involve any damage to the cell, which is of great importance for a potential active substance,” Dräger adds. With a junior associate professorship of the German Center for Infection Research (DZIF) at the University of Tübingen, the bioinformatician works in computer-based systems biology. For this approach, Andreas Dräger, together with his team colleagues Alina Renz and Lina Widerspick, refined an integrated computer model working with the novel coronavirus SARS-CoV-2 and human alveolar macrophages. The latter is responsible for defence against foreign substances in the pulmonary alveoli.
A genome-scale metabolic model
The initial situation in the model is that the virus has invaded the host, in this case, a human alveolar macrophage, and has already reprogrammed it. The model now assumes that the virus wants to produce new virus particles and proliferate. To do this, the virus uses materials from the host and forces the host cells to form new virus components. “Once we understand the composition of the virus, we can run through different scenarios and see how the biochemical reactions in the host cells change during a viral infection.”
Model improved by advanced knowledge about the virus
In the current study, the research group was able to refine their computer model with new information about structural proteins and the SARS-CoV-2 fat metabolism and identify new potential attack points in the nucleotide and lipid metabolisms. In addition, they added information about the threatening virus mutants and analysed whether the already identified weaknesses also applied here. The result was the same for all tested mutants: If guanylate kinase (GK1) was switched off, the proliferation of the virus was stopped.
The team around Andreas Dräger assumes that these findings will form an essential basis for developing inhibitors of the novel coronavirus. Both guanylate kinase 1 and some of the identified drug targets in the nucleotide and lipid metabolisms could respond to antiviral substances without harming humans. “Some inhibitors of the enzyme are already known and, together with our Hamburg-based collaboration partner Dr Bernhard Ellinger from the Fraunhofer IME ScreeningPort (IME), we now want to test already approved inhibitors for their effectiveness against the new coronavirus as soon as possible,” Andreas Dräger explains.