SARS-CoV-2: DZIF scientists and the development of vaccines
Scientists and physicians at the German Center for Infection Research (DZIF) have been closely monitoring and investigating the development of the novel coronavirus SARS-CoV-2 since it first emerged in China. A top priority on the agenda has been to develop a vaccine against the novel coronavirus as quickly as possible.
The DZIF research area “Emerging Infections” is well prepared in the rapid response to outbreaks of this nature. During the Ebola epidemic in 2014, scientists demonstrated the importance of being well prepared for novel virus outbreaks, responding swiftly to significantly drive the clinical development of the Ebola virus vaccine. The DZIF also succeeded in developing a first vaccine against the MERS coronavirus which is closely related to SARS-CoV-2. These previous experiences are now proving advantageous in the face of the current crisis as scientists can now use existing “building blocks”, or so-called vaccine platforms, to develop a vaccine against the novel coronavirus as rapidly as possible.
Vaccine Platform No. 1
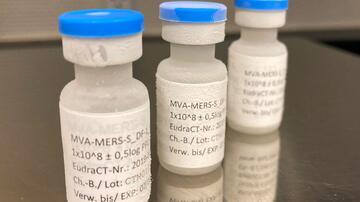
Prof. Gerd Sutter, a virologist at the LMU Munich, is leading a group of scientists in the development of a so-called vector vaccine which uses the “modified vaccinia virus Ankara” (MVA) as a viral vector. MVA was developed at the LMU as a vaccine against smallpox over 30 years ago. MVA viruses are attenuated, and can therefore be used as harmless vectors for other vaccines. At the DZIF, this vector was used to successfully develop a vaccine against the MERS coronavirus which is closely related to SARS-CoV-2. It is currently undergoing clinical trials.
“We hope to be able to use the MVA platform in much the same way as we did for MERS in that we will solely need to insert the genetic coding of a SARS-CoV-2 surface protein,” Sutter explains. “This means that instead of using a part of the MERS coronavirus as a building block, we will combine the vector with a part of SARS-CoV-2.” In order for a vaccine to be effective, it essentially needs to contain specific parts of a virus against which the human body can develop antibodies. Scientists have identified an appropriate coronavirus building block, a so-called spike protein, on the surface of the novel coronavirus. This protein is used by the virus in order to penetrate human cells. The corresponding gene sequence (i.e. the spike protein’s building plan), will be combined with the MVA vector’s genetic information to result in a viral vector which, when administered as a vaccination, is able to penetrate human cells and consequently produce spike proteins. As a result, the immune system can identify these proteins as “foreign” which would stimulate an immune response and the production of specific antibodies and T cells against the spike protein. This in turn can prevent infection with the virus at a later stage.
“We expect the genetic construction of the vaccine and the first steps towards production to be completed in approximately six to eight weeks,” predicts Prof. Stephan Becker from Marburg University. He is the Coordinator of the DZIF research field “Emerging Infections”. This is a huge gain in time when compared to other conventional procedures which use viruses that are capable of replicating for example. However, scientists unanimously agree that even if the first steps towards production go as planned, it will still not be possible to make the vaccine available this year. “Vaccine development is a lengthy, tedious process, particularly the clinical trials for candidate approval. This is not achievable in only just a few weeks,” Becker emphasizes.
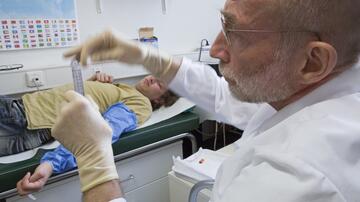
Prof. Marylyn Addo from the University Medical Center Hamburg-Eppendorf (UKE) is leading the clinical trial. She contributed substantially to the development of the Ebola and MERS vaccines, which are still undergoing clinical trials. IDT Biologika will be the company responsible for manufacturing the vaccine in large quantities and it has already developed a producer cell line for large-scale production of the MERS vaccine.
Vaccine Platform No. 2
In order to reach the goal of developing a vaccine as quickly as possible, scientists at the DZIF are in addition conducting research on a second vaccine development platform, alongside MVA. Directed by DZIF scientist PD Dr Michael Mühlebach the measles vaccine is tested as a vector presenting foreign viral proteins. The measles vaccine has been safely used and has been highly effective in billions of vaccinees since the 1960s. Now, this vector is combined with a protein of SARS-CoV-2 acting as target for the immune reaction. These so-called recombinant virus vaccines have already been generated and are currently being produced, and will subsequently be characterised both in vitro and in vivo. “Within half a year our research will demonstrate whether this candidate vaccine based on the measles viral vector is a suitable candidate or not. Following this, other research groups can proceed with developing a corresponding SARS-CoV-2 vaccine,” is Mühlebach’s initial, cautious prognosis. The publication of relevant research findings could enable industrial partners to continue with the development.
CEPI has announced an interest in supporting subsequent development through to the stages of vaccine approval. The “Coalition for Epidemic Preparedness Innovations” is a global partnership between public health authorities, industry and private foundations such as the Bill & Melinda Gates Foundation and aims to give financial support towards any vaccine development projects that are promising.