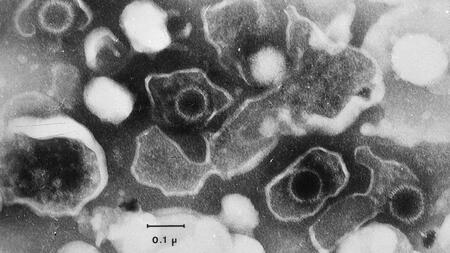
A vaccine candidate against The Epstein-Barr virus
At the DZIF, EBV research has been ongoing for years. Now a promising vaccine candidate is passing from the laboratory to quality assured production. Subsequently, preclinical and early clinical studies can be carried out. The virus causes approximately 200,000 cases of cancer worldwide each year.
Background
The Epstein Barr virus (EBV) is widespread: over 90 percent of the adult population are lifetime carriers of this herpesvirus. Infection is usually asymptomatic, however, this can be misleading as EBV can cause various other diseases such as glandular fever (infectious monoculeosis) and certain types of cancer, particularly in delayed infection. The virus poses a particularly high risk for immunocompromised patients. A vaccine is not yet available.
Development
The history of the vaccine began around 20 years ago at Helmholtz Munich with the development of non-infectious virus-like particles (VLPs) derived from EBV. These VLPs are virtually empty shells made of virus proteins that do not contain the genetic material of the virus, but signal an EBV infection to the immune system and thus trigger immune responses. The DZIF co-funded project yielded positive preclinical proof-of-concept data on the immunogenicity of the vaccine candidate. In addition, the induction of a broad humoral and cellular immune response, which reflects the spectrum of antiviral immunity in humans, has already been demonstrated in animal models.
The start-up EBViously was founded at the beginning of 2023 to enable vaccine production in accordance with the principles of Good Manufacturing Practice (GMP) and thus the start of clinical trials in 2024. The Helmholtz Munich spin-off specialises in novel vaccines based on next-generation virus-like particles. Led by a group of renowned experts in the field of Epstein-Barr virus research around Prof. Wolfgang Hammerschmidt, the company has so far received 9.6 million euros from the Helmholtz Validation Fund (HVF) and the DZIF. Other co-operation partners are the Ludwig-Maximilians-Universität München (LMU) and University Hospital rechts der Isar (TUM MRI).
Partners
- Helmholtz Munich
- Klinikum der Universität München
- Klinikum rechts der Isar der Technischen Universität München (University Hospital rechts der Isar)
- EBViously
- DZIF