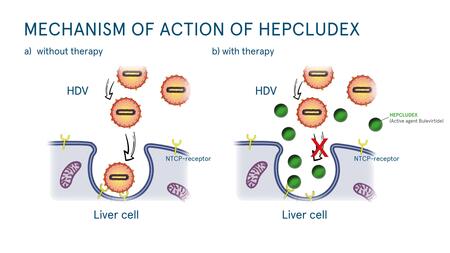
Hepcludex: First drug for hepatitis D has been approved
Professor Stephan Urban and his team developed the first drug for hepatitis D at the Heidelberg University Hospital. So far, there has been no curative treatment – the number of chronically infected patients is high. The drug, which blocks the entry of the hepatitis B and hepatitis D virus into the liver cell, was approved by the European Commission on 31 July 2020 as a drug with the trade name Hepcludex, initially for hepatitis D. The potential uses for hepatitis B are still being investigated.
Background
25 years ago, Stephan Urban discovered the mechanism by which the hepatitis B virus docks to the liver cell. The peptide, which is crucial for the entry of the virus into the cell, was developed over many years into a drug that will initially benefit hepatitis D patients. Hepatitis D infections only occur as an HBV co-infection and lead to liver cirrhosis and liver cancer even more quickly than is the case with HBV infection alone. About 25 million people in the world are suffering from a hepatitis D infection.
Development
Since his appointment as a DZIF professor in 2014, Stephan Urban (Heidelberg University Hospital) has focused on developing a drug based on the blocking peptide he discovered: Myrcludex B. The cooperation with another DZIF team began with the necessary tests in the animal laboratory. Maura Dandri and her team at the University Medical Center Hamburg-Eppendorf succeeded in growing human liver cells in mice. The peptide was also able to prevent infection in mice. The way was paved for clinical trials.
Several phase I and II clinical trials showed that humans tolerate the agent well and that it efficiently prevents the replication of hepatitis B and D viruses. Phase II studies were conducted parallel to the DZIF-funded phase I trial by the licensee and DZIF-contract partner MYR Pharmaceuticals GmbH in Russia. Myrcludex was approved in Russia and the former Soviet Union at the end of 2019. A Phase III study is currently being carried out to investigate the long-term effect of Hepcludex, amongst other things. The scientists also want to test whether the combination of Hepcludex with an immunomodulator can help hepatitis B patients without HDV co-infection.
Partners
- Heidelberg University Hospital
- INSERM
- MYR Pharmaceuticals GmbH (Gilead Sciences, Inc.)
- DZIF